Spinal Cage Pre-clinical Mechanical Assessment - ISO 23089-2
Normative References
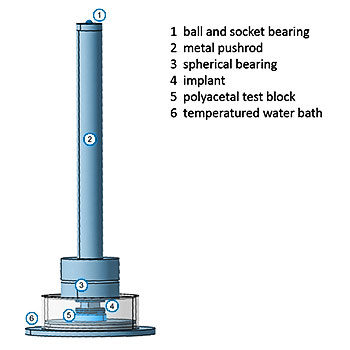
ISO 23089-2: Implants for surgery — Pre-clinical mechanical assessment of spinal implants and particular requirements —Part 2: Spinal intervertebral body fusion devices
ISO 23089-2 bridges the gap between the established ASTM standards for testing intervertebral body fusion devices and the ISO series of standards. In addition, this standard lists further preclinical issues such as coatings, corrosion, and impact behavior.
Optional acceptance criteria for mechanical testing are provided, which result from previously FDA-cleared interbody fusion devices.